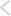The European Medicines Agency (EMA) collaborates with Health Technology Assessment (HTA) agencies, which evaluate medicines and HTA for reimbursement or financing within European Union (EU) Member States. HTA is a multidisciplinary process that assesses the medical, social, economic, and ethical aspects of health technologies in a transparent and unbiased way, aiming to inform patient-centered policies that ensure safety, effectiveness, and value. HTA is rooted in scientific research.
Understanding how medicines are authorized and the product lifecycle is crucial when considering HTA for medicinal products. After conducting clinical trials, pharmaceutical companies submit applications to regulatory authorities for Marketing Authorization (MA) based on safety, quality, and efficacy. For widespread patient access, the product must often be covered by national healthcare systems or insurers, which leads to its inclusion in reimbursement or insurance lists.
Some of the few HTA agencies for pharmaceutical assessment in Europe include:
- France – Haute Autorité de Santé (HAS)
- Germany – Gemeinsamer Bundesausschuss (GBA)
- Scotland – Scottish Medicines Consortium (SMC)
- Sweden – Tandvårds- och läkemedelsförmånsverket (TLV)
Submitting an HTA document for approval in Europe involves several essential steps. First, it is crucial to become familiar with the HTA Regulation (EU 2021/2282), which will take effect on January 12, 2025. In parallel, all necessary clinical, economic, and other relevant data about the health technology must be gathered. The next step involves submitting preliminary information through the Health Technology Assessment Information Technology (HTA IT) platform for joint clinical assessments, which evaluate the relative effects of the technology on health outcomes.
Additionally, requests for joint scientific consultations should be submitted via the platform, enabling refinement of the evidence and methodologies used in the assessment. Once this groundwork is completed, the HTA dossier – including all required documentation – must be prepared and submitted through the HTA IT platform, ensuring compliance with the guidelines and requirements established by the European Commission (EC) and the Member State Coordination Group on HTA (HTACG).
Read more: Revolutionizing Healthcare: The Role of Machine Learning
After submission, the dossier undergoes a joint clinical assessment by the HTACG, which provides a scientific analysis of the clinical evidence. Any feedback or requests for additional information from the HTACG should be addressed promptly. Finally, based on the assessment, the HTACG will issue a report with either recommendations or approval for the health technology.
Figure - Pre-authorization: Marketing Authorization Applications

Source: European Medicines Agency
The Overview of the HTA Development Process in Scotland
The Scottish Medicines Consortium (SMC) serves as the HTA agency for Scotland, providing crucial advice to National Health Service (NHS) Scotland on the clinical and cost-effectiveness of new medicines. Its role is pivotal in ensuring timely access to treatments that offer the greatest benefit, supported by the best available evidence. The SMC evaluates medicines approved by the Medicines and Healthcare Products Regulatory Agency/MHRA or the EMA, including new formulations and indications for established drugs. This evaluation is comprehensive, reviewing clinical trial data, economic assessments, and input from patients and clinicians to ensure that recommended medicines offer maximum patient benefit. The SMC also emphasizes transparency and inclusivity, hosting public meetings to allow stakeholders to voice their opinions and contribute to a fair decision-making process. Once a medicine is approved, it is prescribed within NHS Scotland, ensuring quick access to effective treatments. Additionally, the SMC conducts horizon scanning to identify upcoming medicines, supporting NHS boards in planning and budgeting for new treatments.

Read more: Market Analysis of the PerFuse System for Avascular Necrosis (AVN)
Market Access Strategies for Pharmaceutical Products in Europe
Navigating market access in Europe is a multifaceted challenge, influenced by reimbursement systems, HTAs, and pricing negotiations. Each European country has its own reimbursement process that evaluates a product’s clinical and economic value. HTAs play a critical role in assessing cost-effectiveness and clinical benefits, which directly impact reimbursement decisions. Additionally, pricing negotiations with payers are essential for market entry. Successful companies demonstrate robust evidence during HTA evaluations and engage strategically in pricing discussions to ensure favorable outcomes.
Pricing strategies are crucial for success and are shaped by factors such as manufacturing costs, clinical efficacy, and local economic conditions. Reference pricing, which ties a product’s price to that of similar drugs in other countries, introduces additional complexity. Companies must navigate local market dynamics alongside broader European trends, leveraging innovative approaches such as outcome-based pricing and risk-sharing agreements to align prices with real-world product performance while fostering collaboration with payers.
Entering the European market presents several challenges, including regulatory barriers, intense competition, and diverse market conditions. To address these challenges, companies must collaborate with local experts, conduct thorough market research, and establish strong relationships with healthcare providers and payers. Maintaining flexibility in response to regulatory and market changes is essential for ensuring long-term success.
With a growing emphasis on patient-centricity, companies are increasingly engaging patients in the drug development process and implementing support programs designed to enhance adherence. These patient-focused initiatives not only align with regulatory requirements but also strengthen relationships with healthcare consumers, ultimately positioning organizations for long-term success.
Post-launch strategies are critical to achieving sustained success. Organizations must continuously monitor product performance, evaluate shifts in the market, and actively engage with stakeholders to gather valuable feedback. Effective lifecycle management, including exploring new indications, ensures that a product remains competitive and relevant. Ongoing communication with healthcare professionals, payers, patients, and advocacy groups is essential for maintaining a strong market position and fostering a positive reputation. In the dynamic European pharmaceutical landscape, executing robust post-launch initiatives is indispensable for long-term growth and industry leadership.

Market Trends in Europe for Approval
The European market for drug and HTA approvals is undergoing rapid evolution, shaped by several key trends. In drug approval, there is a noticeable emphasis on accelerated approval pathways, particularly for treatments addressing unmet medical demands, such as rare diseases and innovative therapies. Additionally, regulatory bodies are increasingly integrating real-world evidence (RWE) into their decision-making, utilizing data from actual clinical practice to complement traditional clinical trial data. The EMA is also promoting adaptive pathways, which facilitate earlier patient access to promising new drugs while continuing to gather data on their efficacy and safety. Integration of digital health technologies, like digital biomarkers and telemedicine, is becoming more common in the drug approval process, enhancing the monitoring of drug performance and patient outcomes.
On the HTA side, a significant development is the implementation of a Joint Clinical Assessment (JCA) for oncology drugs as well as Advanced Therapy Medicinal Products (ATMPs) starting in January 2025. By 2030, this will extend to all patented drugs, in-vitro diagnostics (IVDs), and high-risk medical devices, with the aim of harmonizing processes and evidence requirements across EU Member States. This initiative is expected to improve predictability and accelerate patient access to treatments. The JCA process is also likely to raise evidence standards, driven by more experienced HTA bodies, increasing the complexity and rigor of evidence packages required for market access.
Recent methodological updates, including guidelines for quantitative evidence synthesis and subgroup analysis reporting, are shaping this process. Additionally, there is a significant emphasis on stakeholder engagement involving patient organizations, pharmaceutical companies, and academic institutions in the development of HTA guidelines and processes.
Read more: Data Solutions: Navigating the Healthcare Industry through Data-Generated Challenges
Conclusion
Navigating the European pharmaceutical landscape requires a comprehensive understanding of both regulatory and HTA processes. The collaboration between the EMA and HTA agencies is crucial for ensuring timely and equitable patient access to innovative treatments. As HTA practices evolve, the emphasis on transparent, evidence-based evaluations will enhance the decision-making for reimbursement and financing. Pharmaceutical companies must adopt strategic approaches to market access, pricing, and post-launch management to thrive in this dynamic environment. Ultimately, patient-centered policies and efficient market access strategies are essential for long-term success within Europe’s healthcare system.
A leader in the healthcare domain, SG Analytics assists healthcare companies in leveraging the power of information. Contact us today if you are in search of efficient Healthcare solutions to make sound business decisions.
About SG Analytics
SG Analytics (SGA) is an industry-leading global data solutions firm providing data-centric research and contextual analytics services to its clients, including Fortune 500 companies across BFSI, Technology, Media & Entertainment, and Healthcare sectors. Established in 2007, SG Analytics is a Great Place to Work® (GPTW) certified company with a team of over 1200 employees and a presence across the U.S.A., the UK, Switzerland, Poland, and India.
Apart from being recognized by reputed firms such as Gartner, Everest Group, and ISG, SGA has been featured in the elite Deloitte Technology Fast 50 India 2023 and APAC 2024 High Growth Companies by the Financial Times & Statista.